At present, vigorously developing new energy vehicles has become a consensus for countries to achieve energy conservation and emission reduction and cope with climate change. Many countries have raised the development of new energy vehicles to the national strategic level. Major automobile groups in the United States, Europe, Japan and other countries have launched their own development plans. For example, Volkswagen proposed the "2025 Strategy". It is estimated that more than 30 electric vehicles will be launched by 2025, and the sales volume will reach 3 million. Especially since 2016, major auto powers have increased their support for the new energy auto industry:
The German government and industry have provided a total of 1.2 billion euros in subsidies and implemented a special purchase subsidy strategy;
The US government provided a $4.5 billion loan guarantee to promote the construction of electric vehicle infrastructure and invest in the development of high-energy-density batteries.
In this context, as of 2016, the global sales of new energy vehicles exceeded 2 million, of which China accounted for more than 50%, making substantial contributions to energy conservation and global climate change.
However, the current large-scale application of electric vehicles is still subject to many constraints such as driving range, safety, cost, etc. For example, for the driving range of vehicles, simply increasing the number of batteries will cause the whole vehicle to gain weight, which in turn will cause 100 kilometers. The obvious increase in power consumption, followed by the increase in carbon emissions throughout the life cycle, the vehicle price will also rise, so the fundamental solution still needs to significantly improve the performance of all aspects of the battery. Taking the Modle S electric car launched by Tesla in the United States as an example, in order to solve the problem of "mileage anxiety", nearly 7000 3.1 Ah 18650 lithium-ion batteries were used to make the cruising range reach 400 km or more, but the battery weight reached 500 kg, the car's price of up to 79,000 US dollars, to some extent inhibited its promotion in the market.
In China, pure electric drive is used as the technical route. The battery demand of the vehicle is higher, and the more stringent requirements are imposed on the energy density and safety of the battery. Therefore, it is urgent to develop a power battery with high specific energy and high safety. Take into account other properties such as power characteristics, cycle life and cost.
A significant increase in battery performance every time is essentially a major change in the battery material system. From the first generation of nickel-metal hydride batteries and lithium manganate batteries, the second generation of lithium iron phosphate batteries, to the current third-generation ternary batteries, which are widely used and expected to last until around 2020, their energy density and cost are respectively presented. A clear trend of rising and falling steps. Therefore, the battery system used in the next generation of automotive batteries is critical to achieving the battery target of 2020-2025.
In the current various new battery systems, solid-state batteries use new solid-state electrolytes to replace current organic electrolytes and separators, with high safety, high volumetric energy density, and different new high-energy electrode systems (such as lithium-sulfur systems, metals). The wide adaptability of air systems, etc., can further enhance its mass energy density, and is expected to become the ultimate solution for the next generation of power batteries, causing widespread concern among many research institutes, startups and some car companies in Japan, the United States, and Germany.
1 Solid State Battery OverviewConventional lithium-ion batteries use organic liquid electrolytes. In the case of abnormalities such as overcharging and internal short-circuiting, the battery is prone to heat, causing the electrolyte to swell, spontaneously ignite or even explode, posing serious safety hazards. The all-solid-state lithium battery based on solid electrolyte developed in the 1950s, due to the use of solid electrolytes, does not contain flammable and volatile components, completely eliminates the safety hazards such as smoke and fire caused by leakage of batteries. For the safest battery system.
For energy density, the governments of China, the United States and Japan hope to develop prototype devices of 400-500 Wh/kg in 2020 and mass production in 2025-2030. To achieve this goal, the most recognized one is the use of lithium metal anodes. Metal lithium has dendrites, powders, SEI (solid electrolyte interface film) instability, surface side reactions in traditional liquid lithium ion batteries. Many technical challenges, and the compatibility of solid electrolytes with lithium metal makes it possible to use lithium as a negative electrode, thereby significantly achieving an increase in energy density.
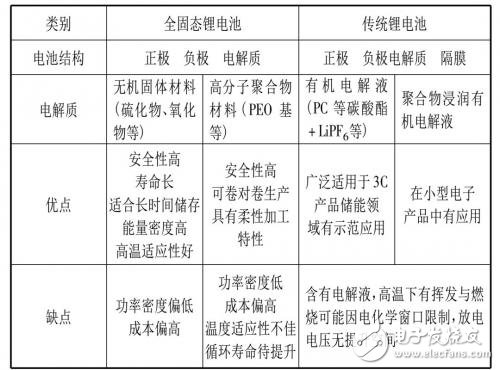
Table 1 Comparison of characteristics of different types of lithium-based batteries
Table 1 compares the traditional lithium battery and the all-solid lithium battery, from which the basic characteristics of the solid lithium battery can be understood. Further, as shown in Table 2, for the desired requirements of automotive battery applications, based on their own characteristics, solid-state battery systems give possible solutions one by one.
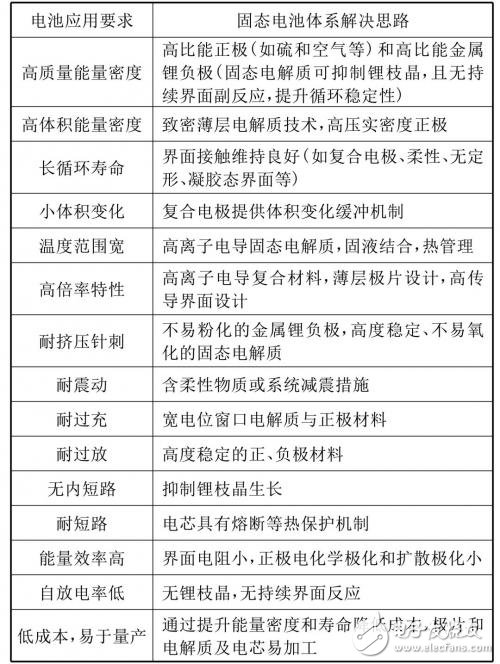
Table 2 Battery application requirements and solid-state battery system solutions
2 Solid-state battery core components - research progress of solid electrolyteFor solid-state batteries, solid electrolytes are a core component that distinguishes them from other battery systems. Ideal solid electrolytes should have:
Maintaining high lithium ion conductivity in the operating temperature range (especially at room temperature);
Neglected or absent grain boundary impedance;
Matching the coefficient of thermal expansion of the electrode material;
Maintain good chemical stability of the positive and negative electrode materials during battery charging and discharging, especially metal lithium or lithium alloy anode;
Electrochemical wide mouth width, high decomposition voltage (> 5.5V vs. Li/Li+);
It is not easy to absorb moisture, the price is low, and the preparation process is simple;
Environmentally friendly.
The following is a detailed discussion of the composition, basic characteristics, technical status, existing problems and modification strategies of different types of solid electrolytes currently under study.
2.1 Polymer solid electrolyte
The polymer solid electrolyte is a kind of lithium ion conductor composed of an organic polymer and a lithium salt, and has the characteristics of light weight, easy film formation, good viscoelasticity and the like. Used in lithium-ion batteries, it can obtain high specific energy, high power, long cycle life battery in a wide operating temperature range, and can prepare batteries in various shapes, making full use of the effective space of electrochemical devices. Polymer lithium-ion batteries can withstand extrusion, collision and temperature and shape changes inside the battery during assembly, use and transportation.
In addition, in addition to its function of transporting lithium ions, the polymer electrolyte can also act as a separator, isolating the positive and negative electrodes, compensating for the volume change of the electrode material during charging and discharging of the battery, and maintaining close contact between the electrode and the electrolyte. The polymer electrolyte can also inhibit the growth of lithium dendrites to some extent, reduce the reactivity between the electrolyte and the electrode material, and improve the safety of the battery. Polymer electrolytes also facilitate large-scale production of batteries in roll-to-roll, which is expected to reduce production costs [1]. Commercially available polymer lithium-ion batteries have been gradually used in electronic devices such as mobile phones, notebook computers, and mobile charging power sources.
A solid polymer battery can be approximated as a solid solution system in which a salt is directly dissolved in a polymer, and its main properties are determined by a combination of a polymer, a lithium salt, and various additives. The choice of lithium salt is actually the choice of anion. In the aprotic, low dielectric constant polymer solvent, the charge density and basic properties of the anion play an important role in the formation of polymer electrolyte.
The ability to form a polymer electrolyte depends on the solvation energy of the cation and the relative size of the salt lattice energy. The larger the lattice energy, the weaker the ability to form a polymer electrolyte with the polymer. The upper limit of the lattice energy of lithium salt is generally considered to be 850 J/mol. Different lithium salts have different lattice energy sizes. The common lithium salt lattice can be ordered [2]: F->Cl->Br->I->SCN- >ClO4-~CF3SO3->BF4-~6AsF6-. In addition to the charge density distribution of the lattice energy and the anion, the dissociation constant of the lithium salt also has a certain influence.
PEO is a typical polymer electrolyte composed of -CH2CH2O- and -CH2CH2CH2O- units. The optimal distribution of ether oxygen atoms in PEO makes it possible to form complexes with various lithium salts. PEO-based polymer electrolytes are also Has been widely studied and applied [3]. For inorganic additives, chemically inert, high specific surface inorganic fillers can improve the thermal stability of polymer electrolytes, inhibit the formation of passivation layers at the electrode interface, provide conductivity and cation migration of electrolytes, etc. The additives are SiO2, Al2O3, MgO, ZrO2, TIO2, LiTaO3, Li3N, LiAlO2 and the like.
At present, the polymer electrolyte has a significant improvement in safety compared with the liquid electrolyte, but it is still necessary to further increase the lithium ion conductivity of the electrolyte, maintaining the mechanical stability and chemical stability of the polymer.
2.2 Inorganic solid electrolyte
The inorganic solid electrolyte takes advantage of its single ion conduction and high stability. It is used in all-solid-state lithium ion batteries, and has the advantages of high thermal stability, non-flammable explosion, environmental friendliness, high cycle stability and strong impact resistance. A wide range of concerns, and is expected to be applied to new lithium-ion batteries such as lithium-sulfur batteries and lithium-air batteries, is the main direction of future electrolyte development.
According to the structure of the material, the inorganic solid electrolyte can be divided into crystalline and amorphous (glassy) two categories, each of which can be divided into oxides and sulfides according to the composition of the elements.
2.2.1 Amorphous (glassy) inorganic electrolyte
The glassy inorganic solid electrolyte has a wide composition change, ion conduction isotropic, relatively low interface impedance, and easy processing into a film, and has a good application prospect in an all solid state battery. According to the composition, it can be divided into oxide system glass electrolyte and sulfide system glass electrolyte, wherein the oxide glass electrolyte has good electrochemical stability and thermal stability, but the ionic conductivity is relatively low, and the sulfide glass electrolyte has high ion conductance. Rate, but poor electrochemical stability, difficult to prepare.
The oxide glass system electrolyte is composed of a network forming oxide (such as SiO2, B2O3, P2O5, etc.) and a network modification (such as Li2O). The network forming oxides are interconnected by covalent bonds to form a glass network, and the network modified oxide Breaking the oxygen bridge in the network allows lithium ions to migrate between their networks. Increasing the conductivity of the oxide glass system electrolyte can be achieved in a number of ways:
First, the amount of network modification can be increased in an appropriate amount. The increase in the content of Li2O by an appropriate amount leads to an increase in the conductivity of the oxide glass electrolyte, and the increase in the content of Li2O to a certain extent leads to an increase in the number of non-oxygen bridge atoms, which can capture lithium ions and thereby reduce oxidation. The glass conductivity can be used to form oxides using a hybrid network. The use of binary or binary networks to form oxides creates a hybrid network effect, increases defect structures in the network, improves transmission bottlenecks in lithium ion conduction channels, and enhances lithium ion conduction. For example, the Li2O-P2O5-B2O3 ternary system glass has a conductivity of 9 & TImes; 10^(-5) S/cm when the lithium ion concentration is 5 mol%.
Silicone Rubber keypads (also known as elastomeric keypads) are used extensively in both consumer and industrial electronic products as a low cost and reliable switching solution.
Silicone rubber refers to the main chain is composed of silicon and oxygen atoms alternately; silicon atoms are usually connected with two organic groups of rubber. Ordinary silicone rubber consists mainly of a silico-oxygen chain with methyl and a small amount of vinyl. The introduction of phenyl can improve the high and low temperature resistance of silicone rubber, and the introduction of trifluoro propyl and cyanide can improve the temperature and oil resistance of silicone rubber. Silicone rubber has good low temperature resistance and can still work at -55℃. After the introduction of phenyl, -73℃ can be reached. The heat resistance of silicone rubber is also very prominent, can work for a long time at 180℃, slightly higher than 200℃ can also withstand a few weeks or longer time is still elastic, instantaneous resistance to high temperature above 300℃. Silicone rubber has good air permeability and oxygen permeability is the highest in synthetic polymers. In addition, silicone rubber also has the outstanding characteristics of physiological inertia, will not cause clotting, so it is widely used in the medical field.
Silicone Rubber Keypads,Membrane Keyboard Switch,Silicone Rubber Waterproof Keypads,Silicone Rubber Numeric Keypad
KEDA MEMBRANE TECHNOLOGY CO., LTD , https://www.kedamembrane.com
